The cooperation of Sanatorium Helios and Genea
Sanatorium Helios is the only clinic in Europe to have signed an exclusive licence agreement for IVF technology with the Australian IVF clinic – Genea, World leading fertility (former Sydney IVF). This agreement was signed in 2002.
The Genea team was led by Tomas Stojanov and Steven McArthur to ensure all protocols, procedures and technologies were consistent between our two clinics. Tomas and Steven were directly responsible for developing world first technologies and procedures, including the Geri incubator and blastocyst biopsy, that generated the best IVF success rates in Australia and around the globe.
Why the license with Genea?
1. Genea, World leading fertility, was established in 1993. Since then, Genea has forged the way for pioneering advances in IVF and today they provide the most encompassing IVF and genetic testing services in Australia (www.genea.com.au).
2. Genea has a strong and long-standing commitment to supporting research – approximately 25 % of pre-tax profits are invested into research, a value that differentiates us from other purely clinical IVF clinics.
3. Genea has developed technologies and equipment used in over 600 clinics worldwide.
4. Genea is the premium leader in pre-implantion genetic testing – former called pre-implantion genetic diagnosis (PGD/PGS). (read more about PGT).
5. Genea has developed so-called mini-incubators, which have revolutionised pregnancy rates. The incubators simulate the natural environment of human fallopian tubes by using low oxygen levels. They also feature just the right amount of carbon dioxide needed to help embryos grow and and maintain a constant temperature (37ºC). Compared to conventional incubators, after opening a mini-incubator, the required concentration of carbon dioxide, temperature and humidity is achieved in a significantly shorter time, thus conditions that help embryos grow are not distorted.
Geri is one of the benchtop incubators used in Sanatorium Helios’ embryology laboratories. Geri benchtop incubator with individually controlled incubation chambers for each patient and time lapse camera allows to continuously monitor embryos, which eliminates the need to open the incubator to check on embryo development. Other time lapse incubators on the market have multiple patients in the one space, meaning even if they had a single step media, embryos would still need to be disturbed when embryologists check other patients’ embryos.

6. Genea has developed its own culture media, which is sold in over 50 countries and used in over 600 clinics worldwide. Development of the third generation of the Genea media suite is underway.
Culture medium, the vital solution that supports embryo development outside the body, has traditionally needed to be replaced at different stages. However, Genea has now developed a continuous culture Geri™ medium, designed specifically for our incubator that is suitable for every stage of embryo development, enabling undisturbed embryo growth.
7. We also have world-leading success with Day 5 blastocyst transfers. Growing an embryo and its subsequent transfer at the blastocyst stage allow selection of the best quality embryos with an expected good viability – it is reliability predicted by extended culture. (read more about extended culture)
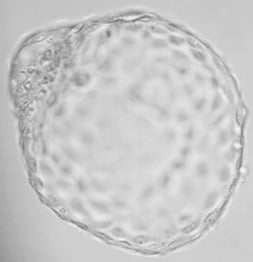
8. Thanks to Genea we are the first clinic in Europe to have started performing blastocyst biopsies. The main advantage of the blastocyst biopsy is that, while the biopsy on Day 3 can examine only one or two cells, the biopsy on Day 5 (6) offers more good quality material (3 to 6 cells) needed to make the examination as accurate as possible.
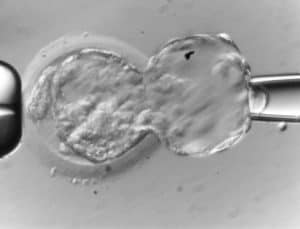
9. Thanks to Genea we are the first clinic in the Czech republic to have started performing vitrification. We have been using the vitrification method since 2007. At present, our vitrification methods are so perfect that the success rate of frozen embryo transfers (KET) corresponds to the success rate of fresh embryo transfers. (more details here)

10. Genea developed a method of freezing oocytes, thanks to which we offer our patients the best chance of preserving their future fertility. In case the partner’s sperm cannot be collected or on the collection day there is no sperm available, the oocytes can be successfully frozen and thawed only when sperm is available. Oocytes can also be frozen if a single woman is worried about her future fertility or in case of cancer treatment. (read more)
11. Thanks to our cooperation with Genea, our success rate attains a very high level.